Learning Objective
- Calculate the molar solubility of a compound in solution containing a common ion.
Key Points
- Adding a common ion decreases the solubility of a solute.
- The common-ion effect can be used to separate compounds or remove impurities from a mixture.
- Different common ions have different effects on the solubility of a solute based on the stoichiometry of the balanced equation.
.Process Oriented Guided Inquiry Learning (POGIL).Increase problem solving, content mastery, and analytical reasoning skills. Common Ion Effect on Solubility. Fractional Precipitation. Common Ion Effect on Acid Ionization. Buffers. Strengths of Acids. Chem 116 POGIL Worksheet - Week 10 Common Ion Effect and Buffers Why? Last week we looked at how to calculate the concentrations of all species and pH or pOH in a solution of a pure acid or base in water, with no additional amounts of the conjugate added. Now, we need to look at the effect of adding extra amounts of the conjugate base or acid.
Terms
- precipitateA solid that exits the liquid phase of a solution.
- precipitateTo come out of a liquid solution into solid form.
- limestoneAn abundant rock of marine and fresh-water sediments; primarily composed of calcite (CaCO₃); it occurs in a variety of forms, both crystalline and amorphous
Adding a Common Ion
If you have a solution and solute in equilibrium, adding a common ion (an ion that is common with the dissolving solid) decreases the solubility of the solute. This is because Le Chatelier’s principle states the reaction will shift toward the left (toward the reactants) to relieve the stress of the excess product. When equilibrium is shifted toward the reactants, the solute precipitates.
Scientists take advantage of this property when purifying water. In areas where water sources are high in chalk or limestone, drinking water contains excess calcium carbonate CaCO3. In the water treatment process, sodium carbonate salt is added to precipitate the calcium carbonate. The very pure and finely divided precipitate of calcium carbonate that is generated is used in the manufacture of toothpaste.
Example:
What is the solubility at 25°C of calcium fluoride (CaF2): (a) in pure water; (b) in 0.10 M calcium chloride (CaCl2); and (c) in 0.10 M sodium fluoride (NaF)?
Solution:
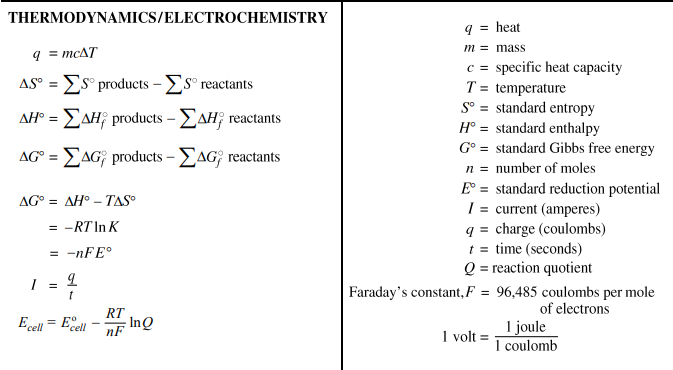
[latex]CaF_2 leftrightarrow Ca^{2+} + 2F^-[/latex]
(a) If the solubility in pure water is s, then
[Ca2+] = s and [F–] = 2s
[latex]K_{sp} = {[Ca^{2+}]}{[F^-]}^2[/latex]
Ksp = s(2s)2 = 4s3 = 3.9 x 10-11
s = 2.1 x 10-4 M
(b) Here the calcium ion concentration is the sum of the concentrations of calcium ions from the 0.10 M calcium chloride and from the calcium fluoride whose solubility we are seeking:
[Ca2+] = 0.10 + s
[F–] = 2s
Ksp = (0.10 + s)(2s)2 = 3.9 x 10-11
Can we simplify this equation? With such a small solubility product for CaF2, you can predict its solubility << 0.10 moles per liter. If our prediction is valid, we can simplify the solubility-product equation:
Common Ion Effect On Solubility Pogil
Ksp = 0.10 (2s)2 = 0.40s2 = 3.9 x 10-11
s2 = [latex]frac{3.90 times 10^{-11}}{0.40}[/latex] = 9.75 x 10-11
s = 9.9 x 10-6 M
If we go back and compare, only 4.7 percent as much CaF2 will dissolve in 0.10 M CaCl2 as in pure water:
[latex]frac{(9.9 times 10^{-6})}{2.1 times 10^{-4}}[/latex] x 100 = 4.7%
Therefore, the approximation that s is small compared to 0.10 M was reasonable.
(c) In 0.10 M NaF:
[Ca2+] = s
[F–] = 0.10 + 2s
since fluoride ions are in NaF as well as in CaF2.
The solubility-product equation is:
Ksp = s(2s + 0.10)2 = 3.9 x 10-11
Again, the equation can be simplified. The 2s term is << 0.10 moles per liter, and therefore:
Ksp = s(0.10)2 = 3.9 x 10-11
s = 3.9 x 10-9 M
This approximation is also valid, since only 0.0019 percent as much CaF2 will dissolve in 0.10 M NaF as in pure water. Fluoride is more effective than calcium as a common ion because it has a second-power effect on the solubility equilibrium.
Common Ion Effect On Solubility Pogil Answers
Show SourcesBoundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
http://www.boundless.com/
Boundless Learning
CC BY-SA 3.0.
http://en.wiktionary.org/wiki/precipitate
Wiktionary
CC BY-SA 3.0.
http://en.wiktionary.org/wiki/limestone
Wiktionary
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/Common_ion_effect
Wikipedia
CC BY-SA 3.0.
http://en.wikibooks.org/wiki/Chemical_Principles/Solution_Equilibria:_Acids_and_Bases%23Common-Ion_Effect
Wikibooks
CC BY-SA 3.0.
http://commons.wikimedia.org/wiki/File:Lithium_hydroxide_with_carbonate_growths.JPG
Wikimedia
Public domain.
Common Ion Effect On Solubility - Displaying top 8 worksheets found for this concept.
Some of the worksheets for this concept are Chem 116 pogil work, Work 23, Common ion effect buffered, Chapter 17 acid base equilibria and solubility equilibria, Example, Solubility and complex ion equilibria, Solubility product work, Saturated.
Found worksheet you are looking for? To download/print, click on pop-out icon or print icon to worksheet to print or download. Worksheet will open in a new window. You can & download or print using the browser document reader options.